Atomic Nucleus : An introduction
2. Nuclear Composition
3. Atomic mass
4. Isobars and Isotones
5. Size of nucleus
1. Discovery of nucleus
- In 1897 J.J. Thomson discovered the electron in the rays emitted from the cathode of discharge tube filled with gas at low temperatures.
- Again 1910 Thomson suggested a model for describing atom , known as 'Thomson's atomic model' which suggests that atom consists of positively charged sphere of radius 10-8cm in which electrons were supposed to be embedded.
- Thomson atomic model failed as it could not give convincing explanation for several phenomenon such as, spectrum
of atoms, alpha particle scattering and many more. - In 1909 Gieger and Marsden employed α-particles (Helium ion) as projectile to bombard thin metallic foil.
- According to Thomson atomic model since all positive charge of atom was neutralized by the negatively charged electrons, there would be rare event for an α-particle to suffer a very large deflection , as expected force of repulsion would not be very strong.
- Surprisingly experiments of Gieger and Marsden showed large deflections of alpha particles that were many orders of magnitude and more common then expected.
- This result of Gieger and Marsden α-particle scattering experiment was explained by Sir Rutherford in 1911.
- Rutherford proposed a new atomic model in which electrons were located at much greater distance from the positive charge.
- Rutherford proposed that all the positive charge , and nearly all the mass of the atom, was concentrated in an extremely small nucleus.
- The electrons were supposed to be distributed around the nucleus in a sphere of atomic radius nearly equal to 10-8cm.
- In explaining this experiment Rutherford made simple assumptions that both the nucleus and α-particles (Helium ion) were point electrical charges and the repulsive force between them is given by Coulomb’s inverse square law at all distances of separation.
- These assumptions made by Rutherford were not valid if α-particle approaches the nucleus to a distance comparable with the diameter of the nucleus.
- From this experiment there emerged a picture of internal structure of atoms and it also confirmed the existence of the atomic nucleus.
- Approximate values for size and electrical charge of nucleus were calculated using data of various scattering experiments.
2. Nuclear Composition
- Atomic nuclei are build up of protons and neutrons.
- Nucleus of hydrogen atom contains only single proton.
- Charge on a proton is +1.6x10-19 C and its mass is 1836 times greater then that of electron.
- Neutrons are uncharged particles and mass of a neutron is slightly greater then that of a proton.
- Neutrons and protons are jointly called nucleons.
- Number of protons in nuclei of an element is equal to the number of electrons in neutral atom of that element.
- All nuclei of a given element does not have equal number of neutrons for example99.9 percent of hydrogen nuclei contains only one proton , some contain one proton and one neutron and a very little fraction contains one proton and two neutrons.
- Elements that have same number of protons but differ in number of neutrons in their nucleus are called ISOTOPES.
- Hydrogen isotope deuterium is stable but tritium is radioactive and it decays to changes into an isotope of helium.
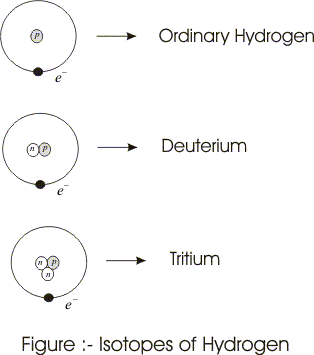
- In heavy water instead of ordinary hydrogen deuterium combines with oxygen.
- Symbol for nuclear species follows the pattern AXZ where
X= Chemical symbol of element
Z= Atomic number of element or number of protons in the nucleus of that element.
A= Mass number of nuclide or number of nucleons in the nucleus. A=Z+N where N is the number of neutrons in the nucleus. - In symbolic form
(1) hydrogen = 1H1 and Deuterium = 2H1
(2) Chlorine isotopes are 35Cl17 and 37Cl17
3. Atomic mass
- Atomic masses refer to the masses of neutral atoms , not of bare nuclei i.e., an atomic mass always includes the masses of all its electrons.
- Atomic masses are expressed in mass units (u).
- One atomic mass unit is defined as one twelfth part of the mass of 12C6 atom.
- So the mass of 12C6, the most abundant isotope of carbon is 12u.
- Value of a mass unit is
1u=1.66054x10-27Kg - We now calculate the energy equivalent of mass unit. We know that Einstein’s Mass-Energy relation is
ΔE=Δmc2
here,
Δm = 1.60x10-27 Kg and
c = 3x108 m/s
therefore
ΔE = (1.60x10-27) x (3x108)2
=1.49x10-10 J
but 1eV = 1.6 x 10-19 J
therefore,
or,ΔE = 1.49 x 10-10 1.60 x 10-19
ΔE = .931 x 109 eV
ΔE = 931 MeV
Thus 1 amu = 931 MeV - Mass of proton is 1.00727663 u which is equal to 1.6725 x 10-27kg or 938.26 MeV.
- Mass of neutron is 1.0086654 u which is equal to 1.6748 x 10-27kg or 939.55 MeV.
4. Isobars and Isotones
- Nuclei with same A but different Z are known as Isobars for example 40K19 and 40Ca20 share same mass number 40 but differs in one unit of Z.
- Although isobaric atoms share same mass number but they differ slightly in their masses.
- This very slight difference in masses of isobaric atoms is related to difference between energies of two atoms since small mass difference corresponds to considerable amount of difference in energies.
- Nuclei with same number of neutrons but different number of protons are called Isotones for example 198Hg80 and198Au79
5. Size of nucleus
- First estimate of size of nucleus was provided by Rutherford scattering experiment.
- In Rutherford’s scattering experiment incident alpha particles gets deflected by the target nucleus as long as the distance approached by the alpha particles does not exceeds 10-14m and Coulomb’s law remains consistent.
- Apart from Rutherford’s scattering experiment various other experiments like fast electrons and neutron scattering experiments were performed to determine the nuclear dimensions.
- Since electrons interact with nucleus only through electric forces so electron scattering experiments gives information on distribution of charge in the nucleus.
- A neutron interacts with nucleus through nuclear forces so neutron scattering provides information on distribution of nuclear matter.
- It was found that the volume of a nucleus is directly proportional to the number of nucleons it contains which is its mass number A.
- If R is the nuclear radius then relationship between R and A is given as
R=R0A1/3
Where value of R0 ≅ 1.2 x 10-15 ≅ 1.2 fm and is known as nuclear radius parameter. - Since R3 is proportional to A this implies that density of nucleas (ρ = m/V) is a constant independent of A for all nuclei.
- The density of nuclear matter is approximately of the order of !17 Kg/m3 and is very large compared to the density of ordinary matter.

No comments:
Post a Comment